Translating…
(Washington D.C.) — Facing growing criticism, the Division of Veterans Affairs talked about Friday that this is rarely any longer going to terminate use of an unproven malaria drug on veterans with COVID-19 however that fewer of its sufferers are now taking it.
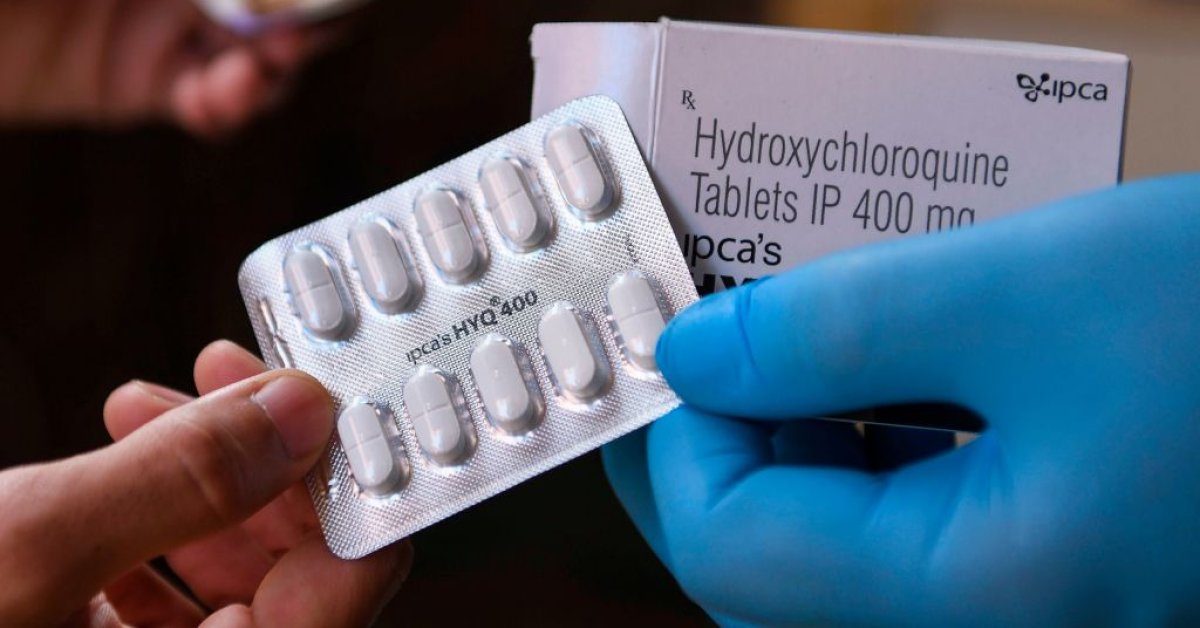
In responses equipped to Congress and got by The Connected Press, the VA talked about it by no methodology “encouraged or terrible” its executive-flee hospitals to utilize hydroxychloroquine on sufferers at the same time as President Donald Trump intently promoted the drug for monthswith out scientific evidence of its effectiveness.
Soundless, it acknowledged that VA Secretary Robert Wilkie had wrongly asserted publicly with out evidence that the drug had been confirmed to succor youthful veterans. The VA, the nation’s largest clinic machine, also agreed extra explore turn out to be wished on the drug and urged its use turn out to be now restricted to extenuating conditions, such as closing-ditch efforts to place a coronavirus affected person’s lifestyles.
Within the first week of May possibly possibly even, 17 sufferers had obtained the drug for COVID-19, according to VA data got by the AP. The department declined to remark what number of sufferers had been handled with hydroxychloroquine for the coronavirus since January, however a fresh evaluation of VA clinic data confirmed that hundreds of veterans had taken it by early April.
“VA has no longer suggested nor terrible the usage of hydroxychloroquine in COVID-19 sufferers and has left those choices to suppliers and their sufferers,” the VA talked about. “Whereas all capsules have the prospective of harmful occasions and a few capsules specifically, like hydroxychloroquine, are identified to have utter dangers, when they’re former rigorously and judiciously, they would possibly perchance well be managed safely.”
As of Friday, 11,883 veterans had been confirmed to be contaminated with the virus and 985 had died, according to VA statistics.
Responding to written questions from Sen. Jon Tester, the pinnacle Democrat on the Senate Veterans Affairs Committee, the department admitted it had no reviews or evidence to support up Wilkie’s claim that hydroxychloroquine had confirmed effectiveness in youthful veterans specifically.
“The usage of hydroxychloroquine for COVID has dropped off dramatically,” the VA talked about.
Trump over and over has pushed the malaria drug hydroxychloroquine with or with out the antibiotic azithromycin, however no natty, rigorous reviews have chanced on them protected or effective for COVID-19, and they’re going to space off coronary heart rhythm complications and various facet outcomes. The Meals and Drug Administration has warned against the drug aggregate and talked about hydroxychloroquine ought to nonetheless entirely be former for the coronavirus in formal reviews.
Two natty observational reviews, each and every engaging around 1,400 sufferers in Original York, no longer too long ago chanced on no profit from hydroxychloroquine. Two unique ones published Thursday within the journal BMJ,one by French researchersand thevarious from China,reached the identical conclusion.
Tester, who obtained VA’s responses this week, talked about he remained angry by the drug’s security.
“Any drug former to take care of sufferers with COVID-19, specifically veterans dwelling with debilitating preexisting stipulations, ought to nonetheless be confirmed protected and effective sooner than it’s administered,” he talked about. “Given fresh reviews from both VA and various hospitals, hydroxychloroquine seems to tumble making an try those requirements.”
Main veterans organizations and Democrats including Senate Minority Leader Chuck Schumer had known as on the VA to display masks why it allowed the usage of an unproven drug on vets. Last week, a whistleblower criticism by former Health and Human Services and products professional Rick Gleaming alleged that the Trump administration wished to “flood” sizzling spots in Original York and Original Jersey with the drug.
Jeremy Butler, CEO of Iraq and Afghanistan Veterans of The United States, talked about Friday that his community turn out to be heartened by the VA’s preliminary explanations and known because it crucial that it start extra miniature print. “It remains pertaining to that it took this long to open to gain solutions to identical previous questions,” he talked about.
The evaluation of VA clinic data, done by just researchers at two universities with VA approval, turn out to be no longer a rigorous experiment. Researchers analyzed scientific recordsdata of 368 older male veterans hospitalized with confirmed coronavirus infection at VA scientific products and companies who died or had been discharged by April 11.
About 28% of veterans who got hydroxychloroquine plus frequent care died, versus 11% of those getting routine care alone.
In its response to Congress, the department equipped copies of about a of its steering issued to VA physicians on hydroxychloroquine from March to May possibly possibly even. It made clear that hydroxychloroquine desires to be regarded as principally for use in clinical trials or when medically appropriate after a stout discussion with the affected person about dangers.
The VA didn’t display masks the conditions wherein veterans within the sizzling evaluation of clinic data got the drug, entirely suggesting that “it is imaginable” they had been prescribed as segment of closing efforts to place a affected person’s lifestyles.
“Basically primarily based entirely on the foundations of affected person-centric care, it can be spoiled to disclaim sufferers gain admission to to hydroxychloroquine under these dire conditions,” VA wrote. It talked about it had followed the design of the Nationwide Institute of Hypersensitivity and Infectious Illnesses by “summarizing the scientific literature relating to unapproved remedies for COVID-19 and making that on hand to VA clinicians of their shared resolution-making with sufferers.” That steering now involves preliminary reviews on remdesivir, which has been federally well-liked for emergency use to take care of COVID-19.
One VA doc dated March 25 notes about a “very miniature cohort reviews” displaying imaginable “beneficial outcomes” however adds “these data have NOT been verified in randomized managed trials and are extremely preliminary.”
The VA acknowledged to Congress that it had placed bulk orders for hydroxychloroquine from Feb. 1 to April 23 for 6.3 million capsules rate $208,000 in anticipation of a imaginable shortage of the drug, however that nearly all of it turn out to be being former for well-liked makes use of, such as treating lupus and rheumatoid arthritis. It talked about it did not have breakdowns.
The department also talked about it planned extra reviews and clinical trials on hydroxychloroquine, including whether it can also abet prevent infection in veterans who had been potentially exposed to COVID-19. It’s far in discussions with Novartis to have some VA products and companies fetch half in a national clinical trial that would possibly perchance discover on the effectiveness of hydroxychloroquine in aggregate with azithromycin in sufferers with moderate and extreme illness.
___
AP Chief Clinical Writer Marilynn Marchione in Milwaukee contributed to this file.
The Coronavirus Temporary.Every thing or no longer it is essential to learn about the worldwide spread of COVID-19

Thanks!
For your security, now we have sent a affirmation email to the take care of you entered. Click the hyperlink to substantiate your subscription and open receiving our newsletters. At the same time as you develop no longer gain the affirmation within 10 minutes, please test your deliver mail folder.
Contact usateditors@time.com.